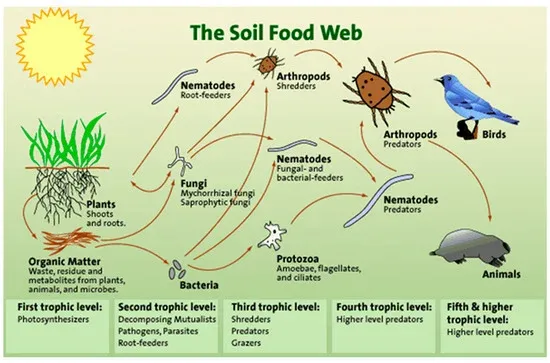
Three of the most determining factors in the use increase of phytosanitary products (PPP) in recent decades have been, on one hand, the soil degradation, its physical and chemical properties as well as the destruction of the biomass (macro and microscopic) that stabilized it. On the other hand, there would be the deterioration of the innate immunity system that plants had to defend themselves from both, biotic and abiotic factors. And as a third factor, no less important, would be the introduction of new pests and diseases from third countries due to poor control by our administrations, which either legislate in a very lax way and/or do not carry out sufficient controls. It is estimated that an average of 10 new harmful organisms are detected every day in the EU territory and the forecast for the coming years is a considerable increase in these events.
There is no doubt that the development of phytosanitary products with specific methods of action against pests and diseases represented a radical change in crop protection, due to their greater effectiveness (shock effect) with small doses. This practice led to the appearance of the phenomenon of resistance, that is, the ability of the pest or disease to survive the doses applied that were previously lethal for its species.
The development of resistance does not occur equally for all classes of chemicals; among the risk factors associated with PPP is the chemical family to which the active substance belongs and its mode of action. In recent years, the number of authorized phytosanitary chemical groups has suffered a great decrease, making it impossible to rotate treatments with different modes of action to avoid resistance.
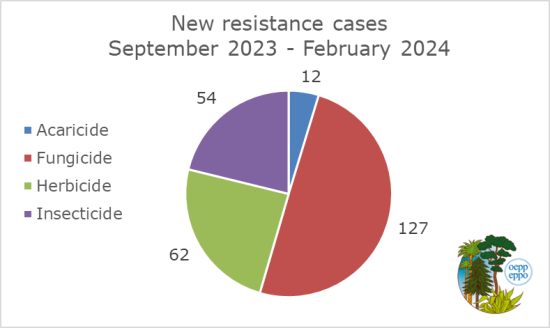
In order to deal with the resistance to PPP in Europe, an EPPO Database on Resistance Cases was created. 26 EPPO countries are participating, and 17 countries entered resistance cases in the database. As of 2024-02-13, a total of 905 cases have been entered and only the resistance cases that have been validated by the EPPO Expert Working Group (EWG) on Resistance to PPP are published in the database and are visible to the public. Since the last EWG meeting in Athens, in September 2023, 255 new cases have been published.
This database was developed initially as a tool for the EPPO Expert Working Group on Resistance and has evolved into a platform to assist EPPO member countries and applicants involved in PPP authorization process. Since the creation of the database a total of 811 cases have been validated. This includes 421 fungicides cases (52%), 196 herbicide cases (24%), 155 insecticide cases (19%) and 39 acaricide cases (5%) have been published. The highest percentage (32%) of cases validated since the creation of the database come from France.
In addition to EPPO, there are two relevant working groups in Europe, such as FRAC which focuses on fungicide and bactericidal resistance prevention strategies and IRAC which focuses on the prevention of resistance to insecticides, acaricides and nematicides.
Among all the techniques available to avoid the appearance of resistance, in addition to all the specific recommendations by FRAC, IRAC as well as the approved instructions for use, the Integrated Pest Management (IPM) must be considered. IPM is the most efficient and environmentally friendly tool for the global protection of crops. IPM constitutes a dynamic process that uses an approach based on ecological systems whose objective is to maintain ecosystem functions and to this end, as a first step, the use of preventive practices in crops is recommended (soil health care, innate immunity of the plant (native species), resistant varieties, crop rotation,…). Next, and in a precise way, the soil and the plant scan be supplied with the fertilizers and biostimulants that best suit the crop, the soil and the rest of the ecosystem.
If pests and/or diseases appear despite these efforts, the use of biocontrol products is recommended in the first instance, with living control organisms, plant extracts, microorganisms-based products, semiochemicals, RNA-based products, among other options. Finally, if control requires the application of a chemical medicine, conventional PPP would be used.
For more information about our services in biocides:
We invite you to contact us
We will be pleased to assist you!
Relevant links: