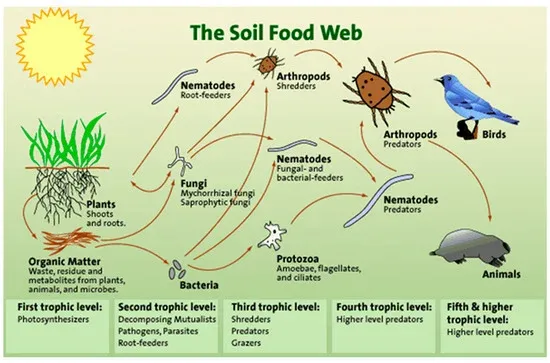
Currently, international organizations, governmental institutions and private companies in the agricultural sector consider that soil health is key to produce food with better nutritional values and, therefore, healthier. The importance of knowing the health of the soil has led to the increasing use of techniques to obtain information on the microorganisms that inhabit it, with the aim of positively influencing its balance and function.
Improving soil health is very important for companies that produce inputs, which is why they have remodeled their product ranges, with the intention of achieving this objective The problem arises when there are several products on the market that claim to produce the same effect on the soil and crops, but do not have scientific validation, which causes the product to lose credibility in the market and there may be a possible deterioration of the company brand image
In order to avoid this situation and to strengthen links with their customers, companies that manufacture inputs must demonstrate the effects that their products are having on the soil in the medium and long term. In addition, knowing the effects is important for future product formulations or screening
There is a technology that solves these problems and is being used by many ag input companies across the globe. Its name is BeCrop® and it was developed by Biome Makers This technology is based on the scientific article “Network Properties of Local Fungal Communities Reveal the Anthropogenic Disturbance Consequences of Farming Practices in Vineyard Soils” which has been validated by the scientific community and published.
The technology is applied in 3 steps The first is soil sampling, this process is validated by Biome Makers and was developed to achieve the highest possible accuracy. The second step is DNA sequencing of soil microorganisms, this process is the most accurate worldwide due to the use of a database of more than 14 million taxonomic references. Finally, advanced bioinformatics and artificial intelligence technologies are applied, transforming the data obtained into a report that reflects information in 2 dimensions: biodiversity, i.e. the microbes that exist in the soil, and functionality, i.e. functions of these microorganisms and their impact on health and nutrient cycles
From this technology, BeCrop® Trials was created, which was developed to analyze all the effects that an input produces on the soil microbiome BeCrop® Trials is a very precise service due to the statistical complexity it uses, because it requires at least 2 locations, 3 replicates and 2 time periods.
Once the analysis is completed, a report is obtained with data on how the product affects, both positively and negatively, the plot analyzed. Companies also get access to the BeCrop® Portal platform, which is used to carry out more detailed analyses
In this project carried out with SeedForward, the effects of the Canola Guard product were tested by performing a BeCrop Trials You can read the real application case by clicking here and you can also watch the complete webinar on the case here. With all this information, problems associated with not knowing the effects of inputs can be avoided. This will help input production companies and their distributors to be able to have scientific reasons that they can provide to their customers to validate commercial claims on soil effects and improve the commercialization of the products.
How to increase sustainable food production in a hotter and drier climate for a constantly growing population while soil is protected?
- Measuring soil quality: physical, chemical, and biological properties of the soil.
- Minimize soil disturbance like tillage and return plant residues to the soil ? rebuilding the stock of soil organic matter, preventing loose soil particles, increasing biodiversity and bioactivity, protecting the network created by mycorrhizal fungi.
- Reduce the use of unnecessary nutrient fertilizers by monitoring regularly the nutrients in soil before making applications.
- When working with biologicals (living organisms) to control a pest, treat a disease or biostimulate plant physiology, the impact in the existing ecosystem should be analysed. The use of biologicals is different than working with chemicals where actions/effects can be more easily predicted. Interaction occurs in a very complex way, adding species can provoke the increase or decrease on existing species, beneficial or not, so unpredictable effects can occur. Partners in the soil change over the time and plants select each time the best microbiome when it is available, so this should be considered on a case-by-case basis to allow biologicals to success in the future.
- When pesticides are needed, the use of less (eco)toxic substances are preferable to avoid impacts on the soil microbiome and the microbially-mediated processes that affect soil functioning.
- We need urgently awareness of good agricultural practises, as soil health has the power to improve plant, human and animal health (“One Health” concept) so we can find together the best way to take advantage of this.
ITEM 3_Strategy to identify combined effects and level of data
ITEM 4_Specific co-formulants
- a)Co-formulants that are approved/no more approved/not approved as pesticide a.s.
- b)Co-formulants that are polymers in PPP
- c)Co-formulants that are UVCBs
- d)Co-formulants that are PFAS
- e)Co-formulants that are formaldehyde releasers
Mycorrhizas
Invisible but essential for the agriculture
It is difficult to estimate when the first plants appeared on our planet Earth. We can imagine that approximately 600 million years ago, seaweed decided to colonize what at that time was a dry soil like the lunar surface. Since then, the first ferns emerged, adapted to the environment and began to establish symbiotic relationships with the fungi that lived in their root system. This relationship materialized in the formation of “supra-organisms” where the fungi obtained carbohydrates from the ferns and these, in return, received water resources, dissolved salts and other biostimulants, allowing both to grow in a mutualistic relationship (symbiosis), where the long-term relationship that they maintain benefited both parties. This community of fungi is known as mycorrhizae. Therefore, there is no doubt that mycorrhizae can be considered an agroecological strategy to optimize crop quality.
We can ask ourselves why our crops are increasingly sensitive to pests and diseases or why they have less quality, among other unwanted characteristics. The problem is that the value of the soil resource and the biomass that lives in it is neither perceived nor valued. In the last decades of exploitation of our crops, there was no awareness of the degradation of the soil, of its properties, including the loss of the mycorrhizal community that colonized the roots of the plants that grew in them. The loss of vegetation cover and increased erosion are signs that this “supra-organism” no longer inhabits those soils.
During the last century, agricultural production focused mainly on the use of mineral fertilizers and chemical products which, to maintain crop yields, involved the use of massive doses that have led us today to the state of degradation that we suffer in the soil. Fortunately, research in recent years in agricultural inputs has been aimed at biological components, such as pests and diseases’ Biocontrol products and Plant Biostimulation, which contribute to increasing soil fertility, as well as improving soil stability and functioning of the entire ecosystem.
For more information about our services in Biocontrol of plant’ pests & diseases and Plant Biostimulants:
We invite you to contact us
We will be pleased to assist you!
Three of the most determining factors in the use increase of phytosanitary products (PPP) in recent decades have been, on one hand, the soil degradation, its physical and chemical properties as well as the destruction of the biomass (macro and microscopic) that stabilized it. On the other hand, there would be the deterioration of the innate immunity system that plants had to defend themselves from both, biotic and abiotic factors. And as a third factor, no less important, would be the introduction of new pests and diseases from third countries due to poor control by our administrations, which either legislate in a very lax way and/or do not carry out sufficient controls. It is estimated that an average of 10 new harmful organisms are detected every day in the EU territory and the forecast for the coming years is a considerable increase in these events.
There is no doubt that the development of phytosanitary products with specific methods of action against pests and diseases represented a radical change in crop protection, due to their greater effectiveness (shock effect) with small doses. This practice led to the appearance of the phenomenon of resistance, that is, the ability of the pest or disease to survive the doses applied that were previously lethal for its species.
The development of resistance does not occur equally for all classes of chemicals; among the risk factors associated with PPP is the chemical family to which the active substance belongs and its mode of action. In recent years, the number of authorized phytosanitary chemical groups has suffered a great decrease, making it impossible to rotate treatments with different modes of action to avoid resistance.
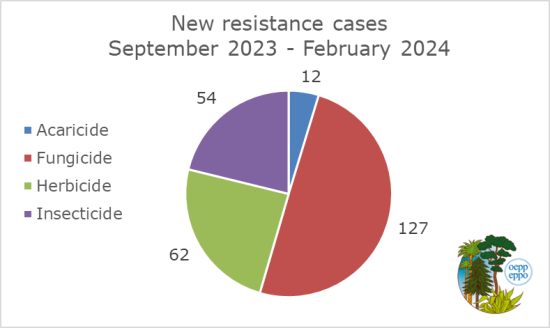
In order to deal with the resistance to PPP in Europe, an EPPO Database on Resistance Cases was created. 26 EPPO countries are participating, and 17 countries entered resistance cases in the database. As of 2024-02-13, a total of 905 cases have been entered and only the resistance cases that have been validated by the EPPO Expert Working Group (EWG) on Resistance to PPP are published in the database and are visible to the public. Since the last EWG meeting in Athens, in September 2023, 255 new cases have been published.
This database was developed initially as a tool for the EPPO Expert Working Group on Resistance and has evolved into a platform to assist EPPO member countries and applicants involved in PPP authorization process. Since the creation of the database a total of 811 cases have been validated. This includes 421 fungicides cases (52%), 196 herbicide cases (24%), 155 insecticide cases (19%) and 39 acaricide cases (5%) have been published. The highest percentage (32%) of cases validated since the creation of the database come from France.
In addition to EPPO, there are two relevant working groups in Europe, such as FRAC which focuses on fungicide and bactericidal resistance prevention strategies and IRAC which focuses on the prevention of resistance to insecticides, acaricides and nematicides.
Among all the techniques available to avoid the appearance of resistance, in addition to all the specific recommendations by FRAC, IRAC as well as the approved instructions for use, the Integrated Pest Management (IPM) must be considered. IPM is the most efficient and environmentally friendly tool for the global protection of crops. IPM constitutes a dynamic process that uses an approach based on ecological systems whose objective is to maintain ecosystem functions and to this end, as a first step, the use of preventive practices in crops is recommended (soil health care, innate immunity of the plant (native species), resistant varieties, crop rotation,…). Next, and in a precise way, the soil and the plant scan be supplied with the fertilizers and biostimulants that best suit the crop, the soil and the rest of the ecosystem.
If pests and/or diseases appear despite these efforts, the use of biocontrol products is recommended in the first instance, with living control organisms, plant extracts, microorganisms-based products, semiochemicals, RNA-based products, among other options. Finally, if control requires the application of a chemical medicine, conventional PPP would be used.
For more information about our services in biocides:
We invite you to contact us
We will be pleased to assist you!
Relevant links:
Desde hace tiempo, existe un importante interés en la evaluación de los peligros de los coformulantes junto con las sustancias activas, debido a su impacto en el uso seguro de los productos fitosanitarios. El personal de la EFSA y de la Comisión Europea ha desarrollado varias propuestas de solución para abordar y apoyar la transparencia y la identificación, los datos y la evaluación de peligros. En esta Parte 1 se comentarán dos de los cuatro puntos debatidos. Los siguientes dos puntos se comentarán en un post posterior (Parte 2):
PUNTO 1_Información sobre la concentración y la función de los coformulantes
La primera cuestión por resolver es cómo identificar plenamente los coformulantes. Se solicitan los requisitos de datos definidos en el Reglamento (UE) nº 284/2013, la composición completa de la mezcla de coformulantes hasta el 100%, información sobre las impurezas contenidas y otros datos fisicoquímicos.
Se han propuesto acciones de seguimiento, como una lista de comprobación de datos para identificar plenamente los coformulantes, y una lista de definiciones de trabajo (glosario) para la evaluación de los productos fitosanitarios y los coformulantes. Estas acciones podrían incluirse en el Documento de orientación sobre cambios significativos y no significativos de los coformulantes (en revisión). Si esto no fuera posible, podría redactarse un documento de orientación específico para los aspectos químicos de la formulación y los coformulantes. Otra propuesta es disponer de un documento independiente para cada coformulante en lugar de un documento dRR para el producto fitosanitario (PPP).
La segunda cuestión es cómo acceder a los datos confidenciales de las composiciones. Una solución a corto plazo es que los Estados Miembros (EM) los compartan en una plataforma específica, o que los proveedores estén directamente en contacto con los EM. Una solución a largo plazo es la creación de una base de datos común de la UE.
La tercera cuestión es definir el coformulante relevante y el coformulante de preocupación que deben tenerse en cuenta para la evaluación de riesgos y supervisarlos durante el estudio de estabilidad. Podrían tenerse en cuenta las nuevas clases de peligro CLP. En este sentido, existe una propuesta de redacción de un documento de orientación con la inclusión de una lista de criterios no exhaustiva.
PUNTO 2_Evaluación del peligro de los PPP/Coformulantes
En principio, debería disponerse de los siguientes datos para evaluar la peligrosidad:
- Genotoxicidad: el cribado de los coformulantes se realizará como se describe en el Documento de orientación sobre la relevancia de los metabolitos en los metabolitos de las aguas subterráneas, 2003.
- Toxicidad aguda (ecotoxicidad): Para los productos fitosanitarios, se dispone de datos de toxicidad aguda (especies no vertebradas) en la mayoría de los casos pendientes de exposición. Faltan datos para las especies acuáticas cuando la aplicación no provoca una contaminación directa de las aguas superficiales. Se propone el siguiente enfoque escalonado: 1) comprobar las propiedades fisicoquímicas y los datos sobre el destino y el comportamiento en el medio ambiente de los productos fitosanitarios/coformulantes y 2) comparar la toxicidad del producto fitosanitario frente a la sustancia activa. Si el PPP es más tóxico, se debería a la toxicidad de los coformulantes (o efecto sinérgico). En el caso de una toxicidad mayor, es necesaria una evaluación de la exposición para poder evaluar el riesgo. En el caso de los coformulantes, podría utilizarse el enfoque por niveles propuesto para la sección de toxicidad en mamíferos. La exposición debe tenerse en cuenta antes de solicitar datos de toxicidad a largo plazo.
- Toxicidad aguda (toxicidad en mamíferos): Para la mayoría de los productos fitosanitarios, se aborda la toxicidad aguda. Aunque dependiendo de la “antigüedad” de los productos, los datos pueden no estar disponibles. En el caso de los coformulantes, en la mayoría de los casos se dispone de datos de toxicidad aguda procedentes de las FDS o de otros marcos jurídicos distintos de los plaguicidas. En la actualidad, los enfoques adoptados por los Estados Miembros difieren enormemente, por lo que será necesario organizar un debate específico con expertos sobre este punto.
- 10Toxicidad a largo plazo (toxicidad en mamíferos): sólo serán aceptables los coformulantes para los que se disponga de todo el conjunto de datos (incluida la toxicidad a largo plazo), a menos que el solicitante desee generar datos. Los coformulantes que no se consideren preocupantes y/o de los que no se necesiten algunos datos pueden incluirse en una lista positiva (basada en el parámetro) o realizar un enfoque escalonado para examinar los coformulantes críticos, tanto los que dispongan de datos como los que no.
Los datos de exención pueden considerarse válidos con una justificación caso por caso y basándose en la opinión de expertos.
Se pueden utilizar diferentes fuentes para la identificación de peligros y los datos que deben tenerse en cuenta: ECHA, EMA, EFSA, Comisión de la UE (ingredientes cosméticos, anexo III) y agencias no pertenecientes a la UE. El solicitante puede utilizar estas fuentes explicando por qué la información extraída es pertinente para la evaluación de riesgos.
Otro punto importante es cómo compartir y armonizar la información y la evaluación de los coformulantes. Existen bases de datos a nivel de los Estados Miembros, basándose en la encuesta de la UE (noviembre de 2022-enero de 2023), que recogen la composición de los coformulantes, existe la base de datos de biocidas de la ECHA sobre coformulantes y también bases de datos de fuera de la UE (por ejemplo, la EPA de EE.UU.). Como solución a largo plazo, se propone crear una base de datos armonizada de la UE a disposición de los Estados Miembros, la Comisión Europea (CE) y las agencias de la UE, con versiones públicas y confidenciales. Como solución provisional, se propone compartir las bases de datos existentes entre los Estados Miembros en CIRCABC / DMS, cuya viabilidad deberán comprobar la CE y la EFSA.
Por otro lado, la evaluación puente de los PPP, los coformulantes alternativos y la evaluación de equivalencia han sido analizadas. Las soluciones propuestas para los productos fitosanitarios consisten en redactar un documento de orientación para definir mejor los criterios (comparación de datos de toxicidad y tipo de formulación, composición y propiedades fisicoquímicas, valores de absorción cutánea, etc.). Las soluciones propuestas para los coformulantes consisten en utilizar el enfoque CLP aplicando el enfoque de lectura cruzada. En cuanto a la ecotoxicidad y la toxicidad en mamíferos, puede ser necesario establecer un puente entre los datos de los coformulantes en caso de que no haya exposición directa al producto fitosanitario, por lo que se ha propuesto la redacción de un documento de orientación.
There is a growing expansion of greener products in the cosmetics industry due to new consumer demands, being a very attractive segment for many companies wishing to have differentiating ingredients and products.
A few years ago, the focus was only on formulations, now the approach is holistic, including packaging, environmental commitments, ethics, social responsibility among other aspects, resulting in more sustainable cosmetic products.
Currently, there is no regulation or standard that indicates when a cosmetic product is considered “natural” or “organic”, unlike food products that have an EU Regulation that specifies the requirements and criteria to be considered within the organic production.
In the absence of regulations on natural and/or organic products, several companies in the sector have joined forces to create certifications for the various cosmetic products and thus establish parameters for evaluation and control. Among the main certifying bodies in Europe are Ecocert and Cosmebio in France, BDIH and Natrue in Germany, Demeter and Soil Association in the United Kingdom. Therefore, in order to obtain some of the organic or natural cosmetics seals, companies must voluntarily undergo certain evaluations and procedures before the certifying companies.
Among the main certifications we can find in the market is “COSMOS”, which has been developed by ECOCERT. On average, products certified by this standard contain 99% ingredients of natural origin, the other ingredients could be from a restrictive list of approved ingredients that are authorized in small proportions. Two classifications can be identified within this standard, first the “COSMOS NATURAL”, which must comply with a minimum of 95 % natural ingredients and the second “COSMOS ORGANIC”, with 95 % organic ingredients and 20 % of the total product which must be organic in order to comply.
Another certification seal that can be found in the international cosmetics market is NATRUE, which establishes a set of strict and transparent requirements for natural and organic cosmetic products. In order for a product to obtain this seal, at least 75% of the products of its brand or sub-brand must obtain certification to be able to use the seal. NATRUE is categorized into two types of products, natural cosmetics and organic cosmetics, for the latter at least 95% of the ingredients must be organic.
In Spain, there is a certification called BioVidaSana, promoted by the Vida Sana Association and bio.inspecta, under the control of Biocertificacion S.L., designed for small companies and traditional manufacturers. This standard has 3 different categories, depending on the percentage of organic or natural ingredients in the product.
There are also other leading organizations in the European Union that are in charge of evaluating greener cosmetics, among them the AIAB, CCPB, ICEA (Italy), ECO Garantie, Bioforum (Belgium) and CosmeBio (France).
Most standards and associations are based on similar fundamentals such as:
- Include in products ingredients that are less harmful to human health, animal health and the environment.
- To be transparent in the market, providing truthfulness in the information transmitted to consumers.
- Increase in the percentage of organic ingredients and harmonization of lists of prohibited ingredients derived from petrochemicals.
- Pursuit of social responsibility by promoting sustainable development and animal welfare.
- Adequate waste management.
- Packaging, labels, commercial material in smaller quantities and with better quality, minimizing unnecessary waste and maximizing the amount that can be recycled.
From Kaeltia Consulting, we advise national and international companies in obtaining greener certifications for cosmetic products, providing support at all stages of the process.
For more information about our services in cosmetics:
CHESAR (CHEmical Safety Assessment and Reporting) Platform is an application under development by the European Chemicals Agency (ECHA) to help companies carry out their chemical risk assessments and document them, serving both the REACH Regulation, where previous CHESAR versions were already used under this framework and, the Biocidal Products Regulation, where the use of this tool is totally new.
Version 1 will be released by mid-2024 (latest info from ECHA, CA-Dec23_Doc.7.2b).
CHESAR Platform will implement the same principles as CHESAR, as the aim is to have an assessment that is structured, harmonized, transparent, and easy to update. The tool will fully integrate EUSES models for environmental assessment, as well as ECETOC TRA models for workers and consumers.
For REACH substances, the tool will support registrants in performing their full chemical safety assessment (for both human health and the environment) and generating their chemical safety reports (CSRs), as currently incorporated in CHESAR.
For Biocides, it will enable initially the environmental risk assessment needed for both active substances and products. The possibility to extend the tool to the human health risk assessment for the active substance and the product will be assessed in the future, also based on the first application experience and the data availability in IUCLID format.
With regard to the environmental risk assessment of biocides, the tool development responds to the current gap in the risk assessment tool roadmap, caused by the fact that the last version of the EUSES software (last updated in 2019) may not accommodate latest updates in emission exposure scenarios and modifications in biocide risk assessments as specified in the Technical Agreements for Biocides (TABs) , including the newly developed emission scenarios, any new Emission Scenario Documents or new guidance.
The intention is that the CHESAR Platform is meant to automatically re-use data on substance properties (if available) from IUCLID and use it as input values for the risk assessment. Once the assessment is finalized, the user could generate (part of) the draft assessment report directly from CHESAR Platform.
Steps and tentative timelines
- Pre-implementation phase – scientific changes – finalized
- Implementation phase – ongoing (almost 300 environmental emission scenarios are being gradually implemented into CHESAR Platform)
- Internal & External Testing phase – in preparation
- Release phase – in preparation: Version 1 of CHESAR Platform will be released in mid 2024 in ECHA Cloud Services, and as desktop application shortly after.
It is foreseen that the tool will be periodically updated (1-2 times a year) to accommodate the new developments on the exposure assessment and risk assessment fronts in biocides in particular, and whenever relevant, also REACH.
The biocidal active substances with anticoagulant rodenticide action (TP14) such as brodifacoum, bromadiolone, chlorophacinone, coumatetralil, difenacum, difethialone and floucoumafen are currently under the renewal process after submitting the corresponding applications to ECHA. The competent authorities of Denmark, Spain, Finland, France, Norway and the Netherlands evaluate applications as evaluating competent authorities.
Since, given the renewal evaluation times, it is likely that the approvals would expire (June 30, 2024) before decisions on their renewals have been made, it is appropriate to delay the expiration date of the approvals long enough to allow applications can be examined. Therefore, on 27 February 2024, the European Commission published Commission Implementing Decision (EU) 2024/734, delaying the expiry date of the approval of all these active substances (a.s.) until 31 December 2026.
Already in October 2023, ECHA informed the Commission that the evaluating competent authorities intend to submit their evaluation reports and conclusions of their evaluations to ECHA in the third quarter of 2024, when an idea of the preliminary conclusions might be available, and we will report on this in due course.
In accordance with the BPR, the approval of these a.s. can only be renewed if they continue to meet the conditions set out in the BPR Regulation as well as the derogating conditions. In this evaluation, the exclusion criteria currently established according to the BPR Regulation for each of these companies must be taken into account:
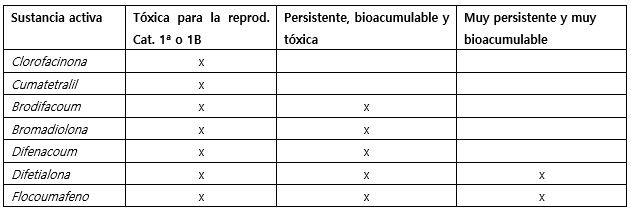
The European Union regulatory framework requires a thorough evaluation to ensure that biocidal products containing these s.a. do not present unacceptable risks to humans or the environment, balancing the benefits of rodent control with potential safety concerns. So, these a.s. are subject to rigorous scrutiny to confirm their safety and environmental compatibility. Therefore, the delay until the end of 2026 allows all parties involved (ECHA, Member States and Stakeholders) enough time to evaluate these substances.
For more information about our services in biocides CLIC HERE or contact us at info@kaeltia.com
We will be pleased to assist you!
On the 13th, 14th and 15th of March, the 17th Plant Health Symposium took place in Seville, where, among others, the following regulatory topics, under the European and national scope, on Plant Health were discussed by representatives of our Authorities and the Industry:
- Dr. Tomás García, scientist at IEGD-CSIC and CEIGRAM, highlighted the importance of the concept “One Health” since the fact that human, plant, animal, and environmental health’s are linked, should not be underestimated and are affected by globalization, tourism, new pests and diseases, among other factors. Likewise, he considered that the legislation that affects products for the control of pests and diseases should be subject to risk-proportionated actions, since the precautionary principle is sometimes used disproportionately and without evidence to support certain measures. Finally, he concluded that the transition towards agroecology can only take place with the involvement of all actors in the supply chain.
- Richard Ramón, representative of the European Commission, focused his presentation on food security and its sustainability, highlighting 14 global megatrends, as the forces that will ultimately cause the change, among which we can mention climate change, degradation of the environment, the limitation of resources, the increase in consumption, among others. He raised the question about how we are going to increase agricultural production that is sustainable and referred us to the Canadian model that has focused its efforts on precision agriculture, biotechnology and digitalization, without a doubt, a model that is worth incorporating into our agricultural practices. He also highlighted the creation of an Observatory of the needs of the primary sector to address the changes that are necessary to avoid unfair practices that harm the sector.
- Valentín Almansa, General Director of Agricultural Production Health, began his speech with the news about the cancellation of the new proposal for the sustainable use of pesticides, a decision very close to the vote of the new European Parliament, and continued his presentation with the results obtained after 2 years of analysis of sales data of (bio)pesticides in Spain. He highlighted that the largest sale, which we could compare to use (there is no data on use, only purchases/sales), corresponds to fungicides and acaricides, to a lesser extent herbicides and insecticides and that this pattern is repeated in the different Regions.
- Emilio Rodríguez, representative of the EU Commission (JRC), commented on the relevance of the fact that, in a context of new emerging pests and diseases, we have fewer products to control them, which is why he focuses on the effort to achieve plant varieties with long-lasting resistance to these pests and diseases. To do this, he focused his presentation on the techniques that are being worked on such as cisgenesis and directed mutagenesis (CRISPR among others). He highlighted the pros and cons of gene modification in plant species, such as the lower use of phytosanitary products as they are more resistant varieties, but with a reduction in the yield of the crop that they are working to improve.
- Dr. José Luis Alonso, representative of the INIA-CSIC, focused his presentation on the integrated pest control as the best pest and disease control strategy, where all types of controls/strategies have a place, always starting with prevention with appropriate agro-practices, followed by products of more natural origin and ultimately, applying chemical control measures when previous therapies have not been effective. Regarding future legislative changes, he reported that they are working on an accelerated procedure for more natural products, something that has been demanded for years by the Biocontrol sector. Efforts are also focusing on the definition of Biopesticides so that, throughout Europe, the same type of products are referred to when this term is used, as well as extending the approval time for low-risk active substances to reduce bureaucracy to administration level. New guidelines will be published by the EU Commission on microbial consortia, RNA-based products, plant extracts, EPPO standards on low-risk risk assessments. For its part, EFSA is working on a new guide to select low-risk active substances from the initial phases, not like now when it is concluded during the process.
- Jacobo Herrero, representative of IBMA Spain, explained to the attendees the proposal that the Association will present to the new European Commission after the cancellation of the new sustainable use proposal where they request: a clear definition of Biocontrol, a working group to evaluate these requests of Biocontrol, eliminate the need to renew this type of products, grant provisional authorizations, make the extension of uses more agile and create a permanent group of evaluation experts.
- Camino García, president of AEFA, for her part, intends to bring to the new European Commission a simple, direct and practical proposal that allows Biocontrol products to be accommodated at the European level with more agility, like the previous speakers said, on the approval of Biocontrol and low-risk active substances, in addition to other procedures like, mutual recognition and parallel trade. In addition, like IBMA, they request a single European entity that evaluates this type of products. Since a modification of the current phytosanitary regulation is not possible, they propose a new legislation only for low-risk products.
- Mª Victoria de la Haza, representative of AEPLA, focused her presentation on some of the techniques and measures that we have at our disposal to reduce and/or mitigate the risks of products that may have certain toxicity for humans. She highlighted the importance of the MAgPIE project initiated in Florida in 2013 as well as the recent draft (March 2024) of the European Compendium to reduce exposure and risk during the use of pesticides and that, in addition, an EFSA guide is expected in the future also in this sense.
For more info about this event: CLIC HERE